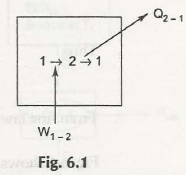
Work can surely be converted into same and equal amount of heat. Therefore, while defining it in mathematical term you can write as W=Q and this indicates direction of energy conversion.
The pulling of a rock on a horizontal rough surface by applying force will help to move the object some distance. But, the friction will create an obstruction while pulling. Once the force has covered the distance it is removed. The block does not have any kinetic energy and comes along with same potential energy that it contains when force start to act.
If we measure the temperature of the block and surface, it is possible to find out that it turns out to be higher compared to the temperature at initial stage. The work performed to move blocks get converted into heat.
In case of passage of current through resistance which may occur in case of electrical work can help to convert into heat. The model operates for electrical heater. These examples actually prove that there is a possibility of having 100% conversion of work into heat.
Links of Previous Main Topic:-
- Introduction to statics
- Introduction to vector algebra
- Two dimensional force systems
- Introduction concept of equilibrium of rigid body
- Friction introduction
- Introduction about distributed forces
- Area moments of inertia in rectangular and polar coordinates
- Mass moment of inertia introduction
- Work done by force
- Kinematics of particles
- Position vector velocity and acceleration
- Plane kinematics of rigid bodies introduction
- Combined motion of translation and rotation
- Rectilinear motion in kinetics of particles
- Work and energy
- Linear momentum
- Force mass acceleration
- Simple stress introduction
- Normal strain
- Statically indeterminate system
- Introduction to thermodynamics
- Statement of zeroth law of thermodynamics with explanation
- Heat and work introduction
- First law of thermodynamics for a control mass closed system undergoing a cycle
- Open system and control volume
Links of Next Mechanical Engineering Topics:-