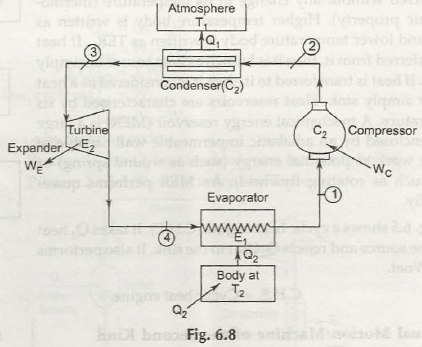
Thermodynamic heat pump and refrigeration cycles are known to be a conceptual and come along with mathematical models. The heat pump is said to be a device that enables to transfer heat from one source that comes with lower temperature to another that comes along with higher temperature by making use of mechanical work or any higher temperature heat sources.
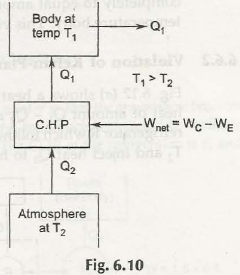
Heat pump can be considered as “heater” in case any objective is used to warm heat sink while “refrigerator” can be called in case any object is used to cool the heat source. In both the cases, operating principles turns out to be identical in nature.
Difference between heat pumps and refrigerator
- Heat transfer:
Both heat pumps and refrigerator may carry out heat from low temperature reservoir to high temperature reservoir. The main objective of refrigerator is to cool the substance while maintaining low temperature in freezer. It also enables to absorb heat and throws it to environment. While heat pump can help to heat up room during winter and maintains when atmospheric temperature is low.
- Location of condenser:
In case of refrigerator it is found outside the refrigerator and stays exposed to atmosphere. With the help of condenser it is possible to push out heat to atmosphere. In case of heat pump, the condenser is found inside the room and finally acts as heating device.
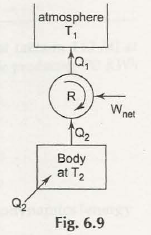
A heat engine is capable of taking heat from any hot substance and finally converts a part into work. It then rejects the rest to cold body. Refrigerator is also known as heat pump but it performs reverse function.
An amount of heat is taken Q2 from cold body and finally amount of work W is done through surrounding and then consider total energy Q1= Q2 that finally gets supplied to hot body in form of heat.
The figure shows the entire process of how it works. In case heat is taken at low temperature T2 and get rejected at high temperature T1 then all parts of these processes can be carried out reversibly.
Links of Previous Main Topic:-
- Statically indeterminate system
- Introduction to thermodynamics
- Statement of zeroth law of thermodynamics with explanation
- Heat and work introduction
- Open system and control volume
- Conversion of work into heat
- Kelvin plank statement of second law of thermodynamics
- Clausius statement of the second law
Links of Next Mechanical Engineering Topics:-