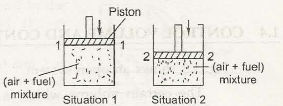
 In the given figure below, Fig. l.l(a) it shows a cylinder having air and fuel of a definite quantity in it, both at the same temperature and pressure. Both have a piston fitted in the container, which is pushed to level 2-2. So in a new position, there will be a new definite volume, pressure and temperature.
In the given figure below, Fig. l.l(a) it shows a cylinder having air and fuel of a definite quantity in it, both at the same temperature and pressure. Both have a piston fitted in the container, which is pushed to level 2-2. So in a new position, there will be a new definite volume, pressure and temperature.
In both the circumstances the volume, temperature and pressure are measured. Thus the state or condition of the systems which is the (air+fuel mixture) is determined by estimating these characters. This analysis is called a macroscopic analysis which we find in engineering thermodynamics.
However, we can conclude that if the analysis is doneby atomic or molecular behaviours of the substance. This study is called microscopic analysis. The macroscopic results are thus also derived from the microscopic analysis.
Links of Previous Main Topic:-
- Linear momentum
- Force mass acceleration
- Simple stress introduction
- Normal strain
- Statically indeterminate system
- Introduction to thermodynamics
- Macroscopic and microscopic point of view
Links of Next Mechanical Engineering Topics:-
- Concepts of systems
- Control volume and control surface
- Homogeneous and heterogeneous systems
- 1 6 1 property
- Thermodynamic equilibrium
- Processes cycles
- Quasi static process
- 1 10 1 reversible process
- Statement of zeroth law of thermodynamics with explanation
- Heat and work introduction
- First law of thermodynamics for a control mass closed system undergoing a cycle
- Open system and control volume
- Conversion of work into heat






















