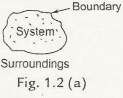
There are three kinds of the system:
- Closed System
- Open System
- Isolated System
1.3.1 Closed System– it is called a closed system when it has an unchanged mass and energy that can flow in or out the boundary. The Fig. 1.2 (b) describes the closed system where no transfers of massoccur.
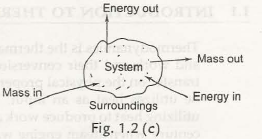
1.3.2 Open System– it is called an open system when the boundary crosses along with the heat and work getshifted. Fig. 1.2 (c) represents an open system.
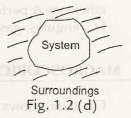
1.3.3 Isolated System– when there is no interaction found observed between the system and surrounding then it is said to be an isolated system. No transfer of mass or energy occurs in this system. So it consists of a fixed mass and fixed power system. Fig. 1.2 (d) shows an isolated system.
Links of Previous Main Topic:-
- Linear momentum
- Force mass acceleration
- Simple stress introduction
- Normal strain
- Statically indeterminate system
- Introduction to thermodynamics
- Macroscopic and microscopic point of view
Links of Next Mechanical Engineering Topics:-
- Control volume and control surface
- Homogeneous and heterogeneous systems
- 1 6 1 property
- Thermodynamic equilibrium
- Processes cycles
- Quasi static process
- 1 10 1 reversible process
- Statement of zeroth law of thermodynamics with explanation
- Heat and work introduction
- First law of thermodynamics for a control mass closed system undergoing a cycle
- Open system and control volume
- Conversion of work into heat