As one of the most important aspects of thermodynamics, entropy is specifically that thermodynamic quantity that represents lack of availability of thermal energy of that system. This energy is specifically required for conversion to that and interprets a certain degree of randomness in the system. If there is a change in the surroundings, there is entropy change, which is specifically driven by heat flow within the system.
There are 2 processes in this regard: Reversible and Irreversible processes.
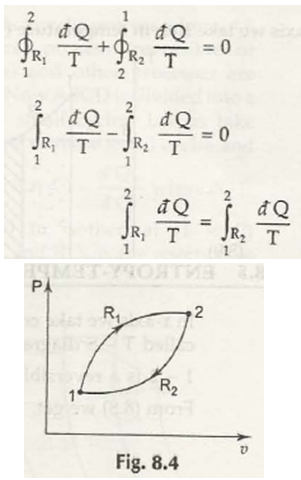
In either case, Clausius theorem is to be applied and it is from here that both the paths are reversed.
Here is a detailed explanation of this process.
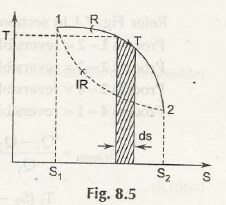
In fig 8.5
Links of Previous Main Topic:-
- Open system and control volume
- Conversion of work into heat
- Introduction to carnot cycle
- Clausius inequality entropy and irreversibility introduction
- Reversible adiabatic paths do not intersect
- Clausius theorem
Links of Next Mechanical Engineering Topics:-
- Entropy temperature plot
- Clausiuss inequality
- Entropy change in an irreversible process
- Principle of increase of entropy
- The degree of irreversibility of and irreversible process
- Summary of first and second law by clausius
- Practical use of entropy principle
- First law and second law combined
- Analysis of thermodynamic equations
- Ideal gas or perfect gas
- Introduction about air standard cycles
- Properties of pure substances introduction
- Vapour compression refrigeration cycle introduction
- Basic fluid mechanics and properties of fluids introduction