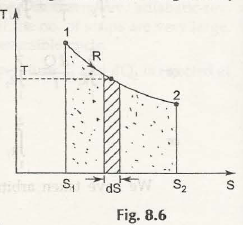
Consider this figure:
From there we get:
dS = d QR / T
d QR = T dS
QR = 2ʃ1 T Ds
= area under the curve 1 – 2
= Heat transfer in the process.
- In x-axis we take entropy (s) and in y-axis we take Kelvin temperature (T).
- This is basically known as called T – 5 diagram.
- T 1 – 2 is a reversible process.
Links of Previous Main Topic:-
- Open system and control volume
- Conversion of work into heat
- Introduction to carnot cycle
- Clausius inequality entropy and irreversibility introduction
- Reversible adiabatic paths do not intersect
- Clausius theorem
Links of Next Mechanical Engineering Topics:-
- Clausiuss inequality
- Entropy change in an irreversible process
- Principle of increase of entropy
- The degree of irreversibility of and irreversible process
- Summary of first and second law by clausius
- Practical use of entropy principle
- First law and second law combined
- Analysis of thermodynamic equations
- Ideal gas or perfect gas
- Introduction about air standard cycles
- Properties of pure substances introduction
- Vapour compression refrigeration cycle introduction
- Basic fluid mechanics and properties of fluids introduction