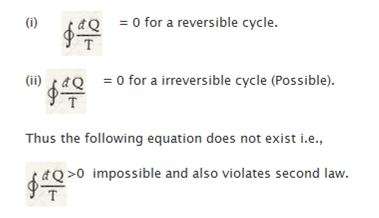Entropy is that thermodynamic quantity that is associated with representing non-availability of thermal energy in a system for conversion into mechanical energy that is termed as a disorder in the system. The entropy changes that are there in a specific system is specifically driven by heat flow as well as signs are determined by that.
When exothermic reactions are taken into consideration, there is a constant temperature within the system that would cause heat to flow into the surrounding area and thereby ensure that the surrounding is rendered positive.
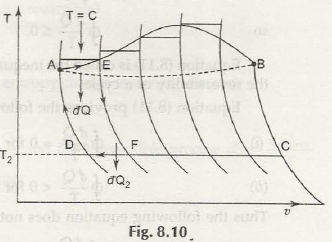
When any such system and its surroundings come across each other in irreversible process, there is an increase in entropy. When entropy of one increases, entropy of another decreases. So, it is taken that this change in entropy in an irreversible process is greater than zero, while in reversible process it is less than zero.
Thus, the equation of entropy for different processes
Links of Previous Main Topic:-
- Open system and control volume
- Conversion of work into heat
- Introduction to carnot cycle
- Clausius inequality entropy and irreversibility introduction
- Reversible adiabatic paths do not intersect
- Clausius theorem
- Entropy
- Entropy temperature plot
- Clausiuss inequality
Links of Next Mechanical Engineering Topics:-
- Principle of increase of entropy
- The degree of irreversibility of and irreversible process
- Summary of first and second law by clausius
- Practical use of entropy principle
- First law and second law combined
- Analysis of thermodynamic equations
- Ideal gas or perfect gas
- Introduction about air standard cycles
- Properties of pure substances introduction
- Vapour compression refrigeration cycle introduction
- Basic fluid mechanics and properties of fluids introduction