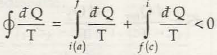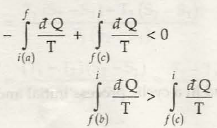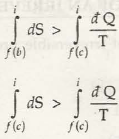Entropy is a specific process in thermodynamic system, where it is used for representing a lack in the thermal energy of the concerned system while it is being converted to mechanical energy. In this case, the whole system functions within a certain sense of randomness, and therefore the associated process has to function in that level.
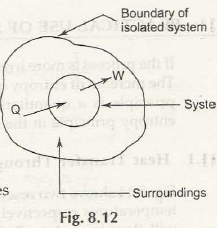
The entropy changes and becomes of the higher levels when it is placed in a secluded system and it is taken to be maximum at the equilibrium point. When there is a total change in entropy, there is enclosing adiabatic surroundings which is zero or at the higher level.
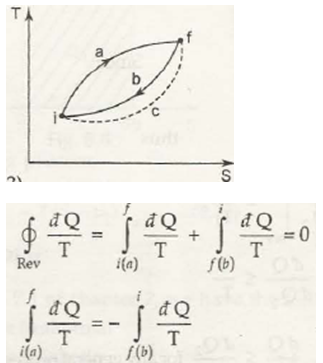
For (a) & (c) cycle
(for a & c irreversible cycle) … (8.14)
From equation (8.13) & (8.14) we can write
(as b is reversible)
(as entropy change is same in band c)
Thus it is proved that the entropy of an isolated system always increases, it can never decrease.
Links of Previous Main Topic:-
- Open system and control volume
- Conversion of work into heat
- Introduction to carnot cycle
- Clausius inequality entropy and irreversibility introduction
- Reversible adiabatic paths do not intersect
- Clausius theorem
- Entropy
- Entropy temperature plot
- Clausiuss inequality
Links of Next Mechanical Engineering Topics:-
- The degree of irreversibility of and irreversible process
- Summary of first and second law by clausius
- Practical use of entropy principle
- First law and second law combined
- Analysis of thermodynamic equations
- Ideal gas or perfect gas
- Introduction about air standard cycles
- Properties of pure substances introduction
- Vapour compression refrigeration cycle introduction
- Basic fluid mechanics and properties of fluids introduction