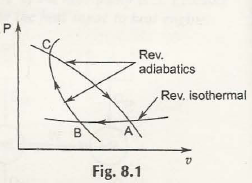
To experiment on this reversible adiabatic path you must consider the following figure:
Points out of the figure:
- Suppose there are two adiabatic paths namely, CA and CB.
- The point in which they intersect is C.
- Let’s assume that there is P, one isothermal path.
- That isothermal path is intersecting in the points A and B with both CA and CB.
After this whole cycle is complete, it will be reversible with paths CA, CB and BC. Here you must notice that there is no heat transfer in the paths CA and CB. The reversibility will be shown in the isothermal path only. Ultimately, it is violating the 2nd law of internal energy.
Result of the experiment:
This experiment shows that assuming two adiabatic paths intersecting with each other is a false point. This reversible cycle is an impossible outcome. By no means will those two adiabatic paths intersect.
Links of Previous Main Topic:-
Links of Next Mechanical Engineering Topics:-
- Clausius inequality entropy and irreversibility introduction
- Reversible adiabatic paths do not intersect
- Clausius theorem
- Entropy
- Entropy temperature plot
- Clausiuss inequality
- Entropy change in an irreversible process
- Principle of increase of entropy
- The degree of irreversibility of and irreversible process
- Summary of first and second law by clausius
- Practical use of entropy principle
- First law and second law combined
- Analysis of thermodynamic equations
- Ideal gas or perfect gas
- Introduction about air standard cycles
- Properties of pure substances introduction
- Vapour compression refrigeration cycle introduction
- Basic fluid mechanics and properties of fluids introduction