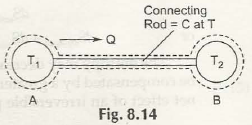
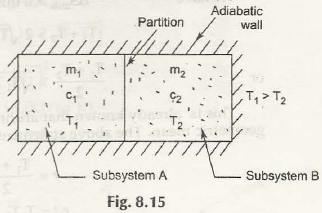
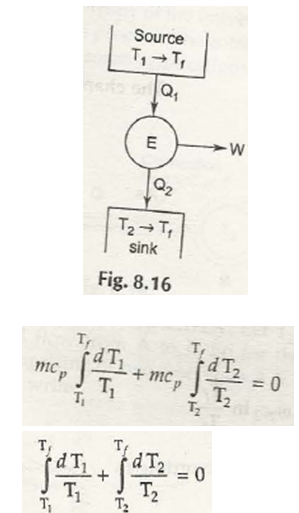
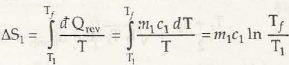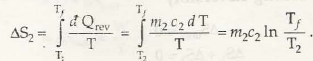Entropy is specifically a thermodynamic process that is associated with showing the lack of thermal energy that is used for converting into mechanical energy. In this case, there is a consistent randomness that is surrounded within the system as well as associated aspects.
The process in this case is both reversible and irreversible. So, when irreversible process is taken into consideration, entropy of universe will increase. Being the quantitative statement of 2nd law, this depends on extent of irreversible process that takes place.
It is very important that while irreversible process is taken, there should be no other associated aspects that has to be functioned.
Links of Previous Main Topic:-
- Open system and control volume
- Conversion of work into heat
- Introduction to carnot cycle
- Clausius inequality entropy and irreversibility introduction
- Reversible adiabatic paths do not intersect
- Clausius theorem
- Entropy
- Entropy temperature plot
- Clausiuss inequality
- Entropy change in an irreversible process
- Principle of increase of entropy
- The degree of irreversibility of and irreversible process
- Summary of first and second law by clausius
Links of Next Mechanical Engineering Topics:-