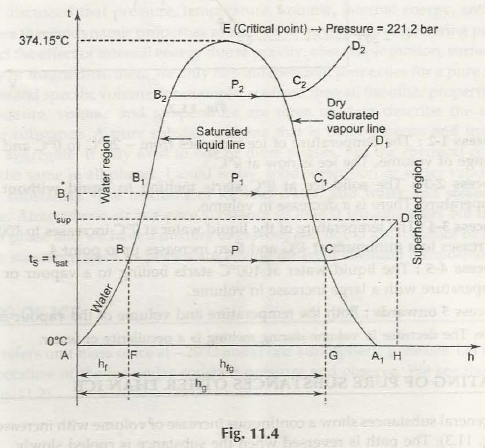
In this case the water at A tends to have a temperature of 0°C and the different stages of it are explained as below
Induction stage- it shows the heating of water up to the boiling temperature. Sensible heat is defined to raise the temperature of water(1 kg mass) from 0°C to boiling point . it is illustrated by is denoted as hf” Sensible heat = mass of water x specific heat x rise in temperature where
1 x 4.2 t s + 273- 273] = 4.2 l 5
Where specific heat of water is taken as 4.2 KJ / kg.
Warming stage- the change of the state from liquid to steam is illustrated by BC. FG is the heat absorbed during this stages and is illustrated by the latent heat of vaporization (h18).
Evaporation stage- the superheating process is shown by CD. During this stage the heat absorbed is GI,I which is known as the heat of superheat. The Line AH is a representation of the total heat that is required to go to the superheated point D
Effect of change on pressure
If the pressure bar is increased to P1, then the boiling temperature is also bound to increase.
point *B1 –the boiling temperature or saturating temperature at P1 bar.
C1- dry saturated steam
Line C1D1 shows the constant pressure process in which the steam is superheated (after C1 point).
In a similar stature, the family of curves is different for various situations. The line A- B-B1- B2 is referred to as the saturated liquid line, as it is the boundary between the water and the steam. On the other hand C2- CcC-A1, is known as dry saturated steam as it is known to formulate the boundary between the dry and the superheated steam. The pressure tends to increase the latent heat of vaporization, and this is the stage where the saturation temperature tends to decrease. It reaches a stage of zero at E where the liquid and dry steam lines meet. At the point E is referred to as a critical point for sure. For steam, critical temperature is 374.15°C and critical pressure is 221.2 bar.
Total heat (enthalpy) of dry saturated steam (point C) is Jz8 = h1 + h/g”
where hg = enthalpy of 1 kg of dry saturated steam
h f = sensible heat of 1kg of water
h fg = enthalpy of vaporisation of 1 kg of dry saturated steam = Latent heat of
steam.
The enthalpy of heating of water upto saturation temperature
h f = c pf (T sat – 273.16)
where c pf = 4.18 kj / kg k.
Wet steam
mg= mass of actual dry steam
m1= mass of water in suspension
m = mass of wet steam
Dryness fraction
x = mg / mg + mf
x is also called the quality of wet steam
for x = 0, it is saturated liquid
for x = 1, it is dry and saturated steam.
0 < x < 1.
Internal energy: h f = u f + P v f
f stands for fluid (water)
u1= hrPv1
for dry steam u g = hg – P v g
g stands for gas (vapour).
(d) sp. enthalpy of wet steam:
h = h1+ xh fg
(e) sp. enthalpy of superheated steam:
h sup = h f+ h fg + C pg U sup – t sat)
c g for steam = 2.1 KJ /kg0k.
t sup – t snt = Degree of superheat.
Specific volume: Specific volume of wet steam
v =v1+x(vg -v1)
= x vg + (1 – x) v1
Charles law when P = C
U sup / T sup = u g / T sat
(g) Specific entropy : specific entropy of wet steam
s = s1 + x (Sg – s1)
(h) Work done during evaporation
=P(vg -v1)
= Pv g as v1= negligible
(i) Work done during evaporation of a wet state
= Px vg.
(j) work done by superheated steam
‘Pv sup.
(k) specific entropy of steam:
The steam is produced at constant pressure. The entropy is assumed to be zero at 0°C.
Change m· spec1· f·1 c entropy= ds = dQ / T
(i) The heat supplied for heating 1 kg of water at constant pressure is given by
D θ = c pf dT
ds = c pf dT / T
Integrating
or s f = c pf in T sat / 273
(ii) The change in entropy during evaporation is given by
S fg = h fg / T sat
(iii) The change in entropy during evaporation is given by
S g = s f + s fg
= c pf in T sat / 273 + h fg / T sat
(iv) Entropy during superheating of steam is given by
(v) Entropy of superheated steam is given by
S sup = s g + s gj
= c pf in T sat / 273 + h fg / T sat
wherecp g = sp heat of superheated steam
= 2.1 KJ/ kg° K
And c pf = 4.18 KJ/ kg0K.
Links of Previous Main Topic:-
- Conversion of work into heat
- Introduction to carnot cycle
- Clausius inequality entropy and irreversibility introduction
- Ideal gas or perfect gas
- Introduction about air standard cycles
- Properties of pure substances introduction
- Heating of pure substances other than ice
- Temperature enthalphy graph formation of steam
Links of Next Mechanical Engineering Topics:-
- Temperature and specific entropy diagram for steam
- The process of thermodynamic and their various properties
- Types of steam table
- Mollier diagram h s axis
- Team power plant simple rankine cycle
- Vapour compression refrigeration cycle introduction
- Basic fluid mechanics and properties of fluids introduction
- Fluid statics introduction
- Manometers measurement pressure