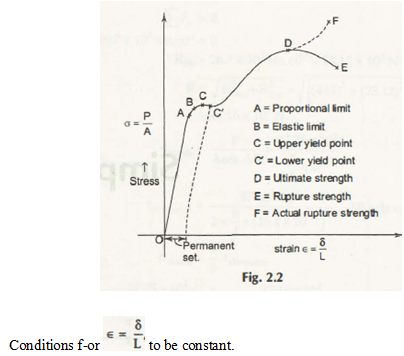
Stress is P/A and Strain is δ/L.
Here, c is considered is constant.
For the mild steel, and for the semi ductile there are some important factors important as –
- The suspended load is axial and thus it must generate a uniform stress.
- The homogeneous material is required.
- Cross-section of the specimen must be constant.
In the above diagram, you can see a number of fundamental concepts related to the curve and it is very important to understand each factor –
- Proportional Limit (A) –
A is the exact point in the diagram where stress ∝ strain.
- Elastic Limit (B) –
In the diagram of stress – strain, B is the elastic limit that indicates that elastic material regains its exact position like before without fixing up any permanent set.
- Yield Point as C or C’ –
Without increasing of stress there is an appreciable deformation. In this diagram, you can see two different points as C and C’ and they are known as yield points.
- Ultimate Stress (D) –
Stress –Strain diagram this is the highest Ordinate.
- Rupture Stress (E) –
At the point where the material or the object breaks is known rupture stress. Calculation of the stress is very important for a proper and nominal diagram. Moreover, it is also important for you to understand that E will be lower than the value of ultimate stress (D).
- Actual Rupture Stress (F) –
It can easily be calculated on the basis of the actual area and this can be taken as the narrow down or the necking phenomena.
Links of Previous Main Topic:-
- Rectilinear motion in kinetics of particles
- Work and energy
- Linear momentum
- Force mass acceleration
- Simple stress introduction
- Normal strain
- Stress strain diagram ductile material mild steel
Links of Next Mechanical Engineering Topics:-
- Axial deformation
- Deformation of a bar due to stress developed
- Poissons ratio
- Shear strain
- Shear stress
- Volumetric strain
- Principle of super position
- Simple strain some definitions
- Working stress and factor of safety
- Statically indeterminate system
- Introduction to thermodynamics
- Statement of zeroth law of thermodynamics with explanation
- Heat and work introduction