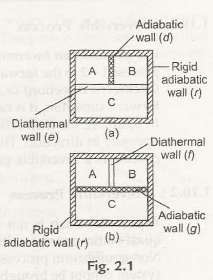
“When there are two systems namely A and B and they have a thermal equilibrium where the temperates are constant along with a third system C. then the other two systems A and B have a thermal equilibrium with each other.”
To make things more clear we take three systems A, B and C as represented in fig 2.1(a). Itis surrounded by a stable adiabatic wall(r). Now A and B systems are paired with the system C with a diathermal wall(e). Now A is detached from B by an adiabatic wall (d). Now the result is A and B gets separated from C which is ina thermal equilibrium condition. It is observed that the system A attains thermal equilibrium with B inspite of it being separated b an adiabatic wall(d). Thus we can conclude that the systems A and B comes to stability even through a diathermal wall(j) and then again decoupled from C by an adiabatic wall (g) as represented in figure 2.1(b).
The zeroth law was already discovered when the first and second law even existed. Thus it is said that the zeroth law leads the first and second laws to form a flexible arrangement.
Links of Previous Main Topic:-
- Introduction to statics
- Introduction to vector algebra
- Two dimensional force systems
- Introduction concept of equilibrium of rigid body
- Friction introduction
- Introduction about distributed forces
- Area moments of inertia in rectangular and polar coordinates
- Mass moment of inertia introduction
- Work done by force
- Kinematics of particles
- Position vector velocity and acceleration
- Plane kinematics of rigid bodies introduction
- Combined motion of translation and rotation
- Rectilinear motion in kinetics of particles
- Work and energy
- Linear momentum
- Force mass acceleration
- Simple stress introduction
- Normal strain
- Statically indeterminate system
- Introduction to thermodynamics
Links of Next Mechanical Engineering Topics:-