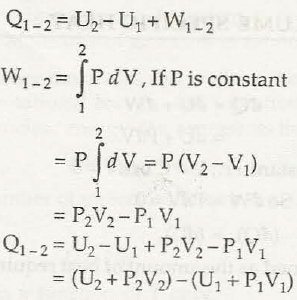Enthalpy would involve both internal energy that is found in a system and energy which is associated along with displacing the surroundings of system. So, in simple terms it can be said that enthalpy can account to heat flow that can happen within system.
It is found that measuring of internal energy can appear to be inaccurate gauge in case of change in energy. Supposedly, a reaction that comes along with constant volume and no work is performed then it would symbolize as:
ΔU=q
But, in case of any open system where there is a chance of gas to escape and enter, then the work is performed as it can be defined in a formula: ΔU≠q. This is because the work is performed.
In case of open systems, the pressure available in a system and surroundings may remain constant. So, the possible change in volume along with constant pressure and internal energy, then enthalpy is used. Enthalpy can be denoted by equation:
∆H = ∆U + P∆V
The law of energy conservation, states that the change in any internal energy would be similar to that of the heat transferred.
Links of Previous Main Topic:-
- Statically indeterminate system
- Introduction to thermodynamics
- Statement of zeroth law of thermodynamics with explanation
- Heat and work introduction
- First law of thermodynamics for a control mass closed system undergoing a cycle
- First law of thermodynamics for a change of state for a control mass closed system
- Different types of stored energy
- The constant volume specific heat
Links of Next Mechanical Engineering Topics:-