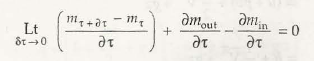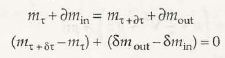The law of conservation of mass may state in closed system, the mass of any system cannot happen over time. In case you have a candle in closed room, you will find that the wax would not be remain in its original form and the mass of wax is present in room, but in different form.
When flame gets lit up the oxygen gas available in the room may react with candle wax and this will finally produce water vapor as well as carbon dioxide gas. If you have the reactants oxygen and wax, then it would be equal to mass of products water and carbon dioxide.
In simple term, the conservation of mass is said that mass of reactants should be equal to that of mass of the products. The law of conservation of mass appears to be quite important when you study about production of chemical reactions.
Chemical manufacturers can help to improve efficiency just by implementing law of conservation of mass to laboratory practices.
Links of Previous Main Topic:-
- Statically indeterminate system
- Introduction to thermodynamics
- Statement of zeroth law of thermodynamics with explanation
- Heat and work introduction
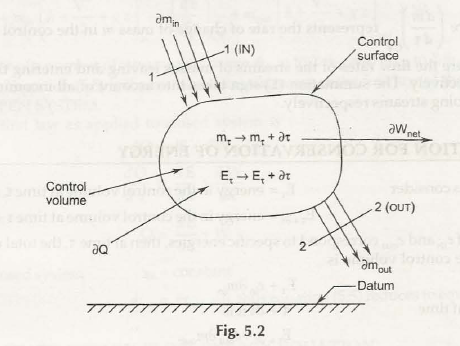
- Open system and control volume
Links of Next Mechanical Engineering Topics:-