What is adiabatic process?
When few systems are subjectedtocertain processes where they are thermally insulated without utilizing the heat transferring procedure, that process is known as anadiabatic process.
Defining reversible process
Quantity of transferred heat entropy change of system
This equation directly defines the meaning of reversible process.
Explaining Reversible adiabatic process
A process that follows both the above procedures (adiabatic and reversible) is known as areversible adiabatic process. This process is isentropic in nature.
Explanation inrelation with thermodynamics
A reversible adiabatic process is stated to be isentropic in nature.Anisentropic process is explained to be adiabatic,and the complete work transfer is frictionless in a system. This process is reversible as matter or heat does not transfer in this process.
Polytropic process
In this process, there is involvement of both work and heat. During the proceedings in case of polytropic processes, computation of heat transfer is mainly based on 2 aspects.
#1
Evaluation of work through steady and open flow process
#2
Assessment of work for closed process with the help of,
- ʃ v d p
- ʃ Pdv
Between both, any of the ones can be used.
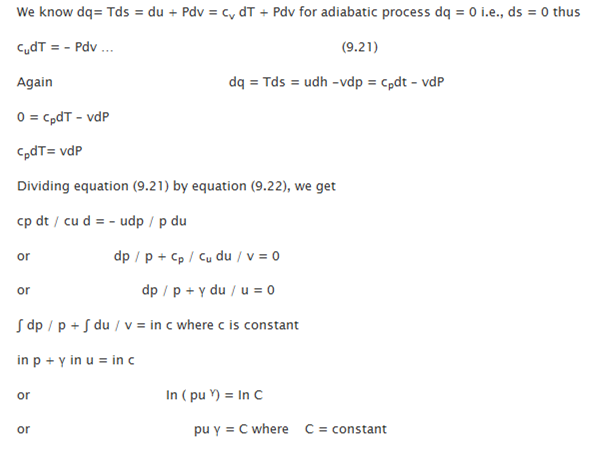
Reversible Adiabatic Process: procedure to detect the governing equation
Links of Previous Main Topic:-
- Open system and control volume
- Conversion of work into heat
- Introduction to carnot cycle
- Clausius inequality entropy and irreversibility introduction
- Ideal gas or perfect gas
- Ideal gas or perfect gas
- Specific heats internal energy and enthalpy of an ideal gas
Links of Next Mechanical Engineering Topics:-
- P v and t s diagrams for pv c
- Equations of state
- Law of corresponding states
- Introduction about air standard cycles
- Properties of pure substances introduction
- Vapour compression refrigeration cycle introduction
- Basic fluid mechanics and properties of fluids introduction
- Fluid statics introduction
- Manometers measurement pressure