Submit Your Homework Stress Free with Our Oxidation of a Cyclic Ketone Assignment Help!
Know vividly about the ketone reactions under the guidance of our experts
Organic chemistry is a sub stream of chemistry. It basically deals with the study of the reactions, properties, and structures of various organic and compounds in other words it is the study of different forms of matter containing carbon atoms. It teaches you about both physical and chemical properties and applies methods to analyze chemical reactivity in order to understand the behavior of the organic matter. So this is really important to know as majority of the compounds around us is organic.
Myhomeworkhelp.com strives to dig deep into the subject matter and clears your concepts with Oxidation of cyclic ketone homework help service.
Baeyer–Villiger oxidation of cyclic ketones:
The Baeyer-Villiger oxidation which is also called Baeyer-Villiger rearrangement basically creates an ester from a lactone or creates a ketone from cyclic ketone. It is an organic reaction where the peroxides or Peroxyacids play as an oxidant. In the year 1899 the reaction was named after Victor Villiger and Adolf Baeyer who established the reaction first. You can opt Oxidation of cyclic ketone assignment help service to easily solve complicated problems on these reactions.
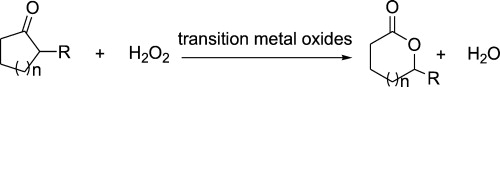
Students can avail our services of Oxidation of cyclic ketone assignment help to solve the projects on this topic quite easily within time and without losing sleep since our subject matter experts are really experienced.
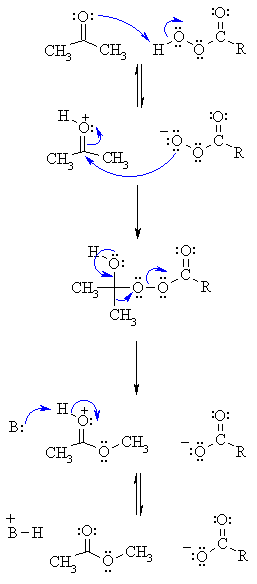
Step wise analysis of THE BAEYER-VILLAGER REACTION
Step1:
An acid/base reaction.
Step2:
The nucleophilic O attacks the carbonyl C by electron shift.
Step3:
The C-C bond is migrated to a new C-O bond.
Step4:
Lastly you get an acid/base reaction revealing the C=O bond and it’s an ester product. Check by Image
All these may seem complicated. But with our Oxidation of cyclic ketone homework help service mostly your basic queries will be answered.
Why we for organic chemistry assignment help
- Guaranteed error-free solution
- We will provide you with 100 % Plagiarism free solution and it is guaranteed to be cent percent original.
- The homework or solution will be self-explaining as we do step by step analysis of the problems with proper explanation and strictly avoid copy-paste from other sources.
- Highly qualified experienced professionals to help
- We offer price quote option to clients. We do your assignments in affordable Price.
- Timely delivery
- Our customer care support works 7 days a week
Nowadays students are already overloaded with too many things on their shoulders. So the main motto of myhomeworkhelp.com is to provide the best help for students so that they can manage their time effectively and also learn the subject from our experts in more depth. So you can try our exclusive Oxidation of cyclic ketone assignment help service to get that desired grade.